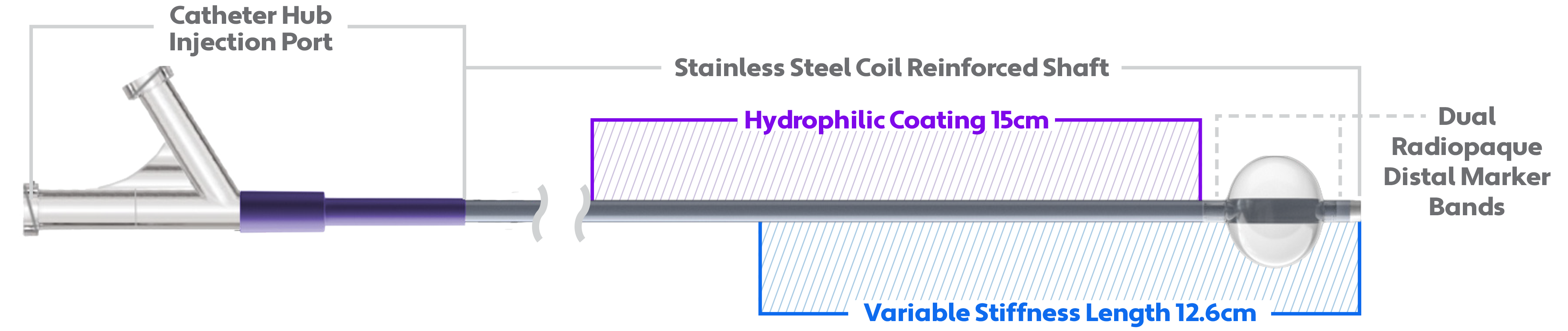
A critical step forward in balloon guide catheter technology.
My particular specifications are unique in this space—which means I’m ideally positioned to serve stroke patients and the caregivers who treat them worldwide. Similar balloon guide catheters have promised superior functionality but fallen short. I was specifically and intentionally designed to banish those shortcomings once and for all.
So how do I deliver? My revolutionary 0.087-inch ID and 8F+ (OD) mean I’m just as suited to facilitate the insertion and guidance of an intravascular catheter as I am in my role as a conduit for clot retrieval devices. And my distinct construction means I deliver proximal support while maintaining distal flexibility. In short, I combine flow control, trackability, support, and access into one great solution.
0.110" OD
Despite my large inner diameter, my outer diameter is small enough to fit in most 8F sheaths—making me suitable to accommodate market-leading closure devices.
0.087" ID
I can accommodate market-leading large bore catheters, maximizing clot capture and improving delivery for the most complex therapies.
11.1mm
Compliant and reliable, my polyurethane balloon diameter is designed to conform to the vessel wall for proximal flow control.